lv sterile filtration | Highly Efficient Large lv sterile filtration Lentiviral vectors (LV) are the most common delivery method for transducing T cells for CAR T therapies, and producing and purifying them are major cost drivers in manufacturing. An industry gold standard of 20% to 40% recovery results in oversized and expensive production batches (1). Effective and consistent . See more China Customized Low Voltage LV Switchgear Manufacturers, Suppliers - FENGYUAN. Home / Products / Switchgear / Low Voltage Switchgear / Details. Low Voltage LV Switchgear GCS devices are suitable for power distribution systems in power plants, petroleum, chemical, metallurgy, textile, high-rise buildings and other industries.
0 · Scalable lentiviral vector clarification
1 · Lentiviral Vector Bioprocessing
2 · Highly Efficient Large
3 · Faster Diafiltration and Ultrafiltration of LV with Vivaflow ® SU
4 · Enhancing the purification of Lentiviral vectors for clinical
This is an authentic LOUIS VUITTON Patent Calfskin Monogram Cherrywood PM in Black. This chic handbag is crafted of glossy patent leather in black with monogram trim. It features a matchingblack patent leather top handle, and an adjustable monogram shoulder strap.
Scalable lentiviral vector clarification
Lentiviral vectors (LV) are the most common delivery method for transducing T cells for CAR T therapies, and producing and purifying them are major cost drivers in manufacturing. An industry gold standard of 20% to 40% recovery results in oversized and expensive production batches (1). Effective and consistent . See moreHere we present a scalable, closed workflow for LV clarification by filtration. The three-stage filtration system allows for effective clearance of LV producing cells as well as debris and aggregates approximately 0.45 µm and larger with minimal to no loss of . See more
dolce gabbana light blue living stromboli 75ml
All work was performed in collaboration with CCRM through funding from FedDev Ontario and Cytiva at the Centre for Advanced Therapeutic Cell Technologies (CATCT), Toronto, . See moreSterile filtration is typically the final step of the downstream process, wherein the viral vectors, . Sterilizing grade filtration of concentrated and diafiltered LV was performed with .
This report describes a simpler method using two tangential flow filtration (TFF) steps to .Lentiviral vectors (LV) are widely used for gene therapy and genetically modified cell therapies, .
Effective and consistent purification strategies for LV are a serious challenge for four reasons: the labile nature of the virus; the need to physically segregate LV from the cells from which they bud; the need to remove host cell DNA (HCD) and protein (HCP); and the use of 0.2 µm sterile filtration for high concentrations of 0.08 to 0.12 µm .Sterile filtration is typically the final step of the downstream process, wherein the viral vectors, having been purified, concentrated and formulated are passed through a fine filter to remove any adventitious agents such as bacteria or fungi while maintaining LV titres. Sterilizing grade filtration of concentrated and diafiltered LV was performed with Supor® EKV Mini Kleenpak™ syringe filters (Pall Corporation) washed with Milli Q water and equilibrated with buffer (minimum of 20 L/m 2 of each) before use. Filtration was .
This report describes a simpler method using two tangential flow filtration (TFF) steps to concentrate liter-scale volumes of LV supernatant, achieving in excess of 2000-fold concentration in less than 3 hours with very high recovery (>97%). Lentiviral vectors (LVs) have been increasingly used as a tool for gene and cell therapies since they can stably integrate the genome in dividing and nondividing cells. LV production and purification processes have evolved substantially over the last decades. Laboratory-scale production of LV is typically achieved by ultracentrifugation of filtered culture medium from human HEK293T cells transiently cotransfected with a combination of 3 or more packaging and one transfer vector plasmid. The sterile filtration step was successfully performed and allowing a global virus recovery up to 45%. This work unravels strategies to enhance lentivirus vector purification process which may accelerate the development of therapeutic products based on .
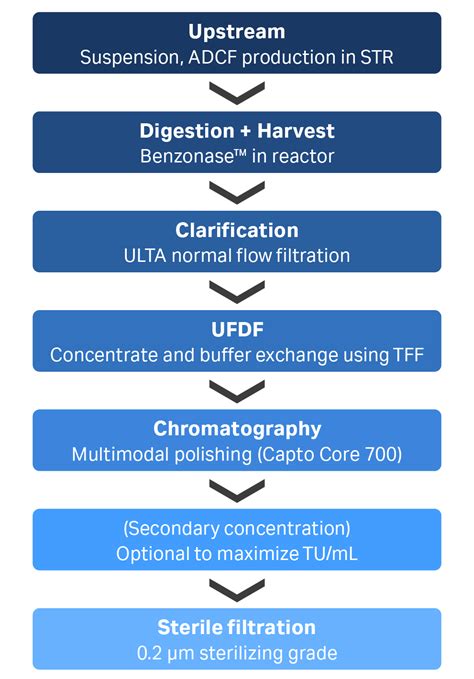
Lentiviral vectors (LV) are widely used for gene therapy and genetically modified cell therapies, with many clinical trials in the pipeline. A typical downstream processing workflow for LV comprises clarification, purification, diafiltration, ultrafiltration, and finally sterile filtration.Sterile filtration is at the end of the workflow. Groups across the board have recorded historically low recoveries at this step. In addition to the pain points in the downstream process, there are challenges with the virus itself.2 filters: Ensure sterile filtration using sterilizing-grade heterogeneous double-layer filters available in 0.8 and 0.45 μm pre-filter and a 0.2 μm final filter membrane. Virosart Media: Mitigate viral contamination using a viral retention filter designed for cell culture media.Effective and consistent purification strategies for LV are a serious challenge for four reasons: the labile nature of the virus; the need to physically segregate LV from the cells from which they bud; the need to remove host cell DNA (HCD) and protein (HCP); and the use of 0.2 µm sterile filtration for high concentrations of 0.08 to 0.12 µm .
Sterile filtration is typically the final step of the downstream process, wherein the viral vectors, having been purified, concentrated and formulated are passed through a fine filter to remove any adventitious agents such as bacteria or fungi while maintaining LV titres. Sterilizing grade filtration of concentrated and diafiltered LV was performed with Supor® EKV Mini Kleenpak™ syringe filters (Pall Corporation) washed with Milli Q water and equilibrated with buffer (minimum of 20 L/m 2 of each) before use. Filtration was .This report describes a simpler method using two tangential flow filtration (TFF) steps to concentrate liter-scale volumes of LV supernatant, achieving in excess of 2000-fold concentration in less than 3 hours with very high recovery (>97%).
Lentiviral vectors (LVs) have been increasingly used as a tool for gene and cell therapies since they can stably integrate the genome in dividing and nondividing cells. LV production and purification processes have evolved substantially over the last decades. Laboratory-scale production of LV is typically achieved by ultracentrifugation of filtered culture medium from human HEK293T cells transiently cotransfected with a combination of 3 or more packaging and one transfer vector plasmid. The sterile filtration step was successfully performed and allowing a global virus recovery up to 45%. This work unravels strategies to enhance lentivirus vector purification process which may accelerate the development of therapeutic products based on .Lentiviral vectors (LV) are widely used for gene therapy and genetically modified cell therapies, with many clinical trials in the pipeline. A typical downstream processing workflow for LV comprises clarification, purification, diafiltration, ultrafiltration, and finally sterile filtration.
Sterile filtration is at the end of the workflow. Groups across the board have recorded historically low recoveries at this step. In addition to the pain points in the downstream process, there are challenges with the virus itself.
Lentiviral Vector Bioprocessing
Highly Efficient Large
dolce gabbana light blue for her macy'
Faster Diafiltration and Ultrafiltration of LV with Vivaflow ® SU

China ABC Accessories catalog of Round Aluminum Bracket Anchor Bracket for LV-ABC Cables (CSDY10), Hook for Fix The Anchor Clamp in Metal, Steel, Wooden Pillar or The Wall of Building (CS-10) provided by China manufacturer - Wenzhou .
lv sterile filtration|Highly Efficient Large